Our research
How the drug candidate AVX001 works
Reccura Therapeutic’s drug candidate AVX001
AVX001 is a highly potent and selective small molecule blocking the activity of the key enzyme cPLA2α. The molecule is mimicking the natural substrate of the enzyme and belongs to a chemically class of modified polyunsaturated fatty acids. AVX001 is developed by Professor Berit Johansen at NTNU in Trondheim in close collaboration with Professor Lars Skattebøl at University of Oslo.
AVX001 is formulated in a cosmetically attractive gel.
Key strengths of the AVX001 gel:
- Clinically validated drug candidate
- Well characterized mode-of-action
- Strong evidence for treating inflammatory driven cancers
- Skin friendly with excellent safety profile and limited skin reaction
- Cosmetically attractive gel formulation with high delivery of the drug candidate into the skin
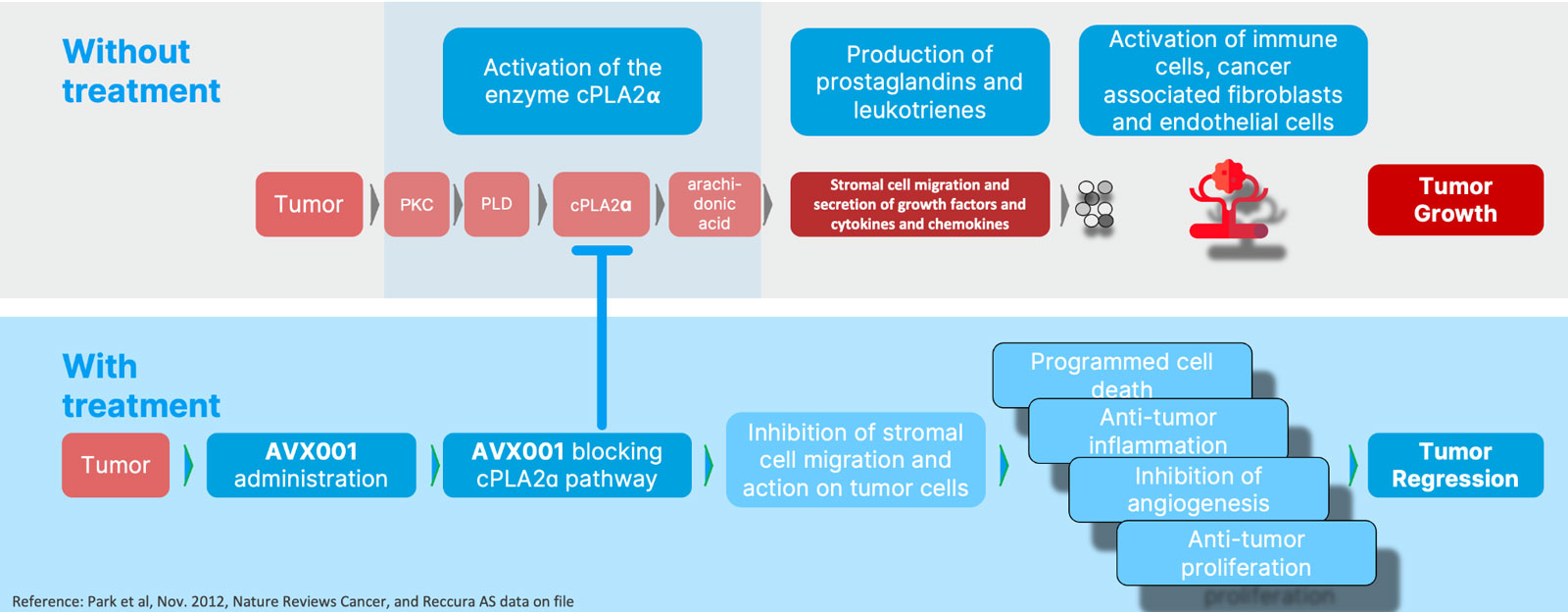
A novel and well characterized mode of action targeting the cPLA2𝝰 enzyme
Reccura Therapeutic is based on pioneering research on the cPLA2α enzyme and represent a potential paradigm shift for the treatment of inflammatory driven cancers.
AVX001 efficiently and selectively blocks the cPLA2α enzyme activity leading to significantly reduced levels of arachidonic acid which in turn reduces tumor growth by affecting several key hallmarks of cancer development, including programmed cell death, anti-tumor inflammation, inhibition of angiogenesis, and anti-tumor proliferation.
The role of cPLA2α in cancer
There is substantial evidence that cPLA2α plays a key role in the development of cancer and inflammation, two aspects that play a key role in the development of the inflammatory driven diseases AK and BCC. Furthermore, high cPLA2α levels correlate with metastasis and poor prognosis of several cancers. Even poor treatment outcomes of existing treatments have been shown to be correlated with high cPLA2α levels, and inhibiting cPLA2α in combination with existing standard of care treatments, for example radiation, increases the treatment response in preclinical models.cPLA2𝝰 as a key biomarker for poor prognosis in canc
cPLA2α as a key biomarker for poor prognosis in cancer
High levels of cPLA2𝝰 are associated with poor prognosis for patients
Cytosolic Phospholipase A2 group IVA enzyme (cPLA2α) is an emerging and promising new target for treating cancers. The figure below shows that elevated cPLA2α expression is associated with poor prognosis and survival of cancers (red curves), as compared with patients with no or low levels of cPLA2α expression (green curves).
Positive data
Promising results in completed clinical trials
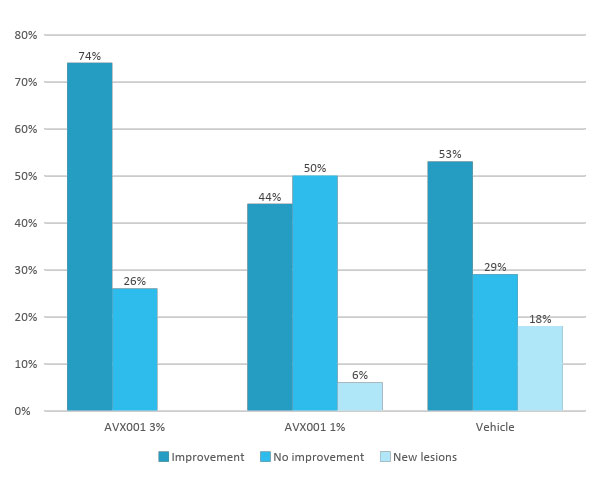
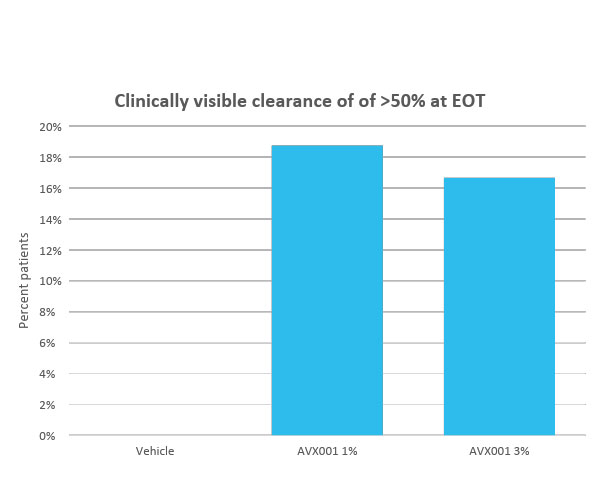
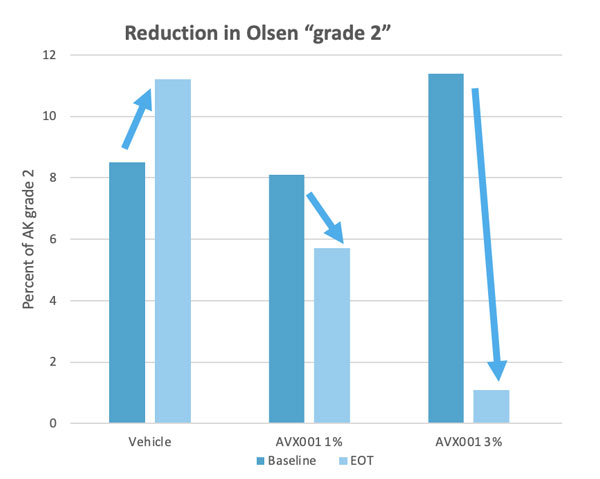
Clinical safety and efficacy studies have been completed in four phase I/IIa clinical studies assessing AVX001. Two studies in a topical ointment formulation for the treatment of mild-to-moderate psoriasis patients, and two in a gel formulation for the treatment of atopic dermatitis and AK. In the latter, we conducted the Copenhagen Actinic Keratosis Study (COAKS), a 12-week single-center, randomized, vehicle-controlled, double-blind, hybrid clinical trial in adults with multiple AK lesions Olsen grade 1 or 2. In this study the AVX001 topical gel-formulation was found to be safe and tolerable, and effectiveness was observed in for the highest dose. From the three other studies AVX001 was also found to be safe and effectiveness was shown in treating psoriasis patients.
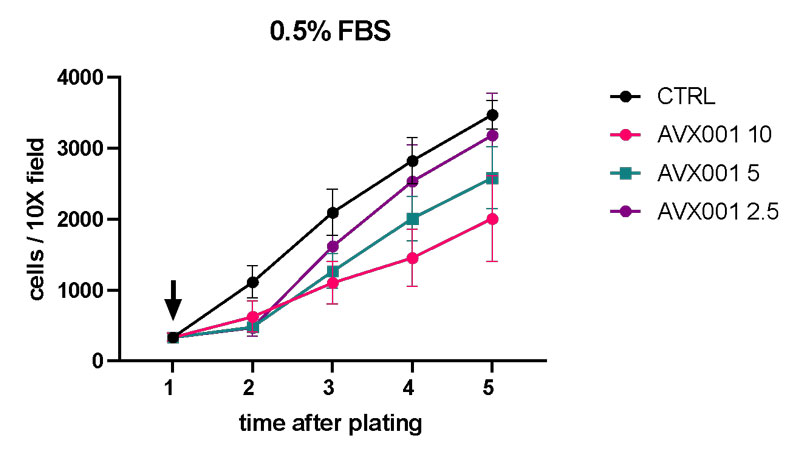
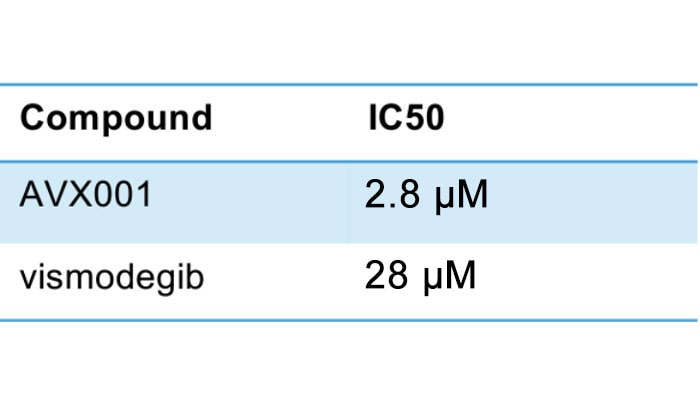
Promising data of AVX001 in BCC
AVX001 inhibits BCC cell viability 10 times more potent than the marketed vismodegib
Researchers and publications
Reccura Therapeutics builds on a strong Norwegian and North American scientific foundation
AXV001 was developed by prof. Berit Johansen at NTNU in Trondheim, Norway and Professor Leif Skattebøl at Univerity of Oslo, Norway. The groundbreaking research of the key enzyme cPLA2α and its central role in inflammation and cancer has been performed in close collaboration with prof. Edward Dennis at University of California, San Diego and Professor Joseph Bonventre at Harvard Medical School, Boston.
The research of cPLA2α and the role of AVX001 in AK and BCC has been investigated by Professor Berit Johansen, NTNU and Professor Merete Hædersdal at the Danish Research Center for Skin Cancer, University of Copenhagen.

Professor Berit Johansen
– International Expert in cPLA2α Compounds and inventor of the AVX-compounds
Department of Biology, Faculty of Natural Sciences, Norwegian University of Science and Technology (NTNU).
Dr. Johansen is a Professor and Ph.d. in Molecular Genetics. She has a vast experience in molecular biology, inflammation, cancer research and cPLA2. Dr. Johansen did hold distinguished positions at: UCLA, US; University of Uppsala, Sweden; NTH, Department of Technical Biochemistry; at Unigen, University of Trondheim, Norway; Institute of Molecular Genetics, University of Göttingen, Germany; Biogen Research Corporation, Cambridge, MA; Department of Chemistry and Biochemistry, University of California, San Diego, CA., and Harvard Medical School, Boston, MA, USA.

Professor Edward A. Dennis
– International Expert in PLA2
Department of Chemistry Biochemistry, University of California, San Diego, USA.

Professor Joseph Bonventre
– International Expert in Renal Diseases and PLA2
Brigham and Women’s Hospital, Renal Unit, Harvard Medical School, Boston, USA.
Dr. Bonventre is Chief of the Renal Unit and Director of the Bioengineering Division at Brigham and Women’s Hospital and has had a long-standing interest in various aspects of cellular injury and repair mechanisms in the kidney with a special emphasis on the role of inflammation, biomarkers and stem cells. One of his key focus research areas has and is still PLA2, contributing to the understanding of cPLA2α as a target.

Professor, Dr. med., PhD, MD Merete Hædersdal
– International Expert in Skin Cancer
Danish Research Center for Skin Cancer, University of Copenhagen, Denmark.




Key publications
Ortner, V. K., Johansen, B., Kilov, K., Mondragón, A. C., Duvold, T., Kihl, J.R. Zibert & Haedersdal, M. The Copenhagen Actinic Keratosis Study (COAKS). A decentralised clinical trial to evaluate tolerability, safety and efficacy of daily field-directed topical treatment with cytosolic phospholipase A2 inhibitor, AVX001, in participants with actinic keratosis: protocol for a randomised controlled phase I/IIa trial. BMJ open, 12(10), 2022, e061012.
Ashcroft FJ, Mahammad N, Midtun Flatekvål H, Jullumstrø Feuerherm A, Johansen B. cPLA2α Enzyme Inhibition Attenuates Inflammation and Keratinocyte Proliferation. Biomolecules. 2020 Oct 2;10(10):1402. LINK
Mahammad N, Ashcroft FJ, Feuerherm AJ, Elsaadi S, Vandsemb EN, Børset M, Johansen B. Inhibition of Cytosolic Phospholipase A2α Induces Apoptosis in Multiple Myeloma Cells. Molecules. 2021 Dec 9;26(24):7447. LINK
Feuerherm AJ, Dennis EA, Johansen B. Cytosolic group IVA phospholipase A2 inhibitors, AVX001 and AVX002, ameliorate collagen-induced arthritis. Arthritis Res Ther. 2019 Jan 21;21(1):29. LINK
Omland SH, Habicht A, Damsbo P, Wilms J, Johansen B, Gniadecki R. A randomized, double-blind, placebo-controlled, dose-escalation first-in-man study (phase 0) to assess the safety and efficacy of topical cytosolic phospholipase A2 inhibitor, AVX001, in patients with mild to moderate plaque psoriasis. J Eur Acad Dermatol Venereol. 2017 Jul;31(7):1161-1167. LINK
Kim E, Kim J, Maelandsmo GM, Johansen B, Moestue SA. Anti-angiogenic therapy affects the relationship between tumor vascular structure and function: A correlation study between micro-computed tomography angiography and dynamic contrast enhanced MRI. Magn Reson Med. 2017 Oct;78(4):1513-1522. LINK
Kim E, Tunset HM, Cebulla J, Vettukattil R, Helgesen H, Feuerherm AJ, Engebråten O, Mælandsmo GM, Johansen B, Moestue SA. Anti-vascular effects of the cytosolic phospholipase A2 inhibitor AVX235 in a patient-derived basal-like breast cancer model. BMC Cancer. 2016 Mar 7;16:191. LINK
Sommerfelt RM, Feuerherm AJ, Jones K, Johansen B. Cytosolic phospholipase A2 regulates TNF-induced production of joint destructive effectors in synoviocytes. PLoS One. 2013 Dec 12;8(12):e83555. LINK
Huwiler A, Feuerherm AJ, Sakem B, Pastukhov O, Filipenko I, Nguyen T, Johansen B. The ω3-polyunsaturated fatty acid anaolgues AVX001 and AVX002 directly inhibit cytosolic phospholipase A(2) and suppress PGE(2) formation in mesangial cells. Br J Pharmacol. 2012 Dec;167(8):1691-701. LINK