Plans
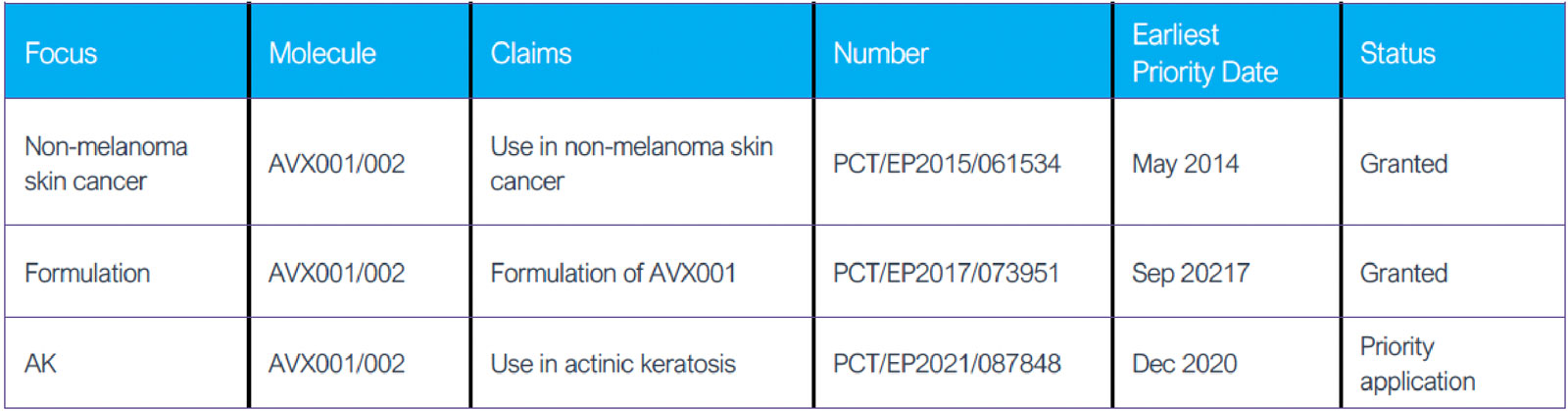
Strong patent protection
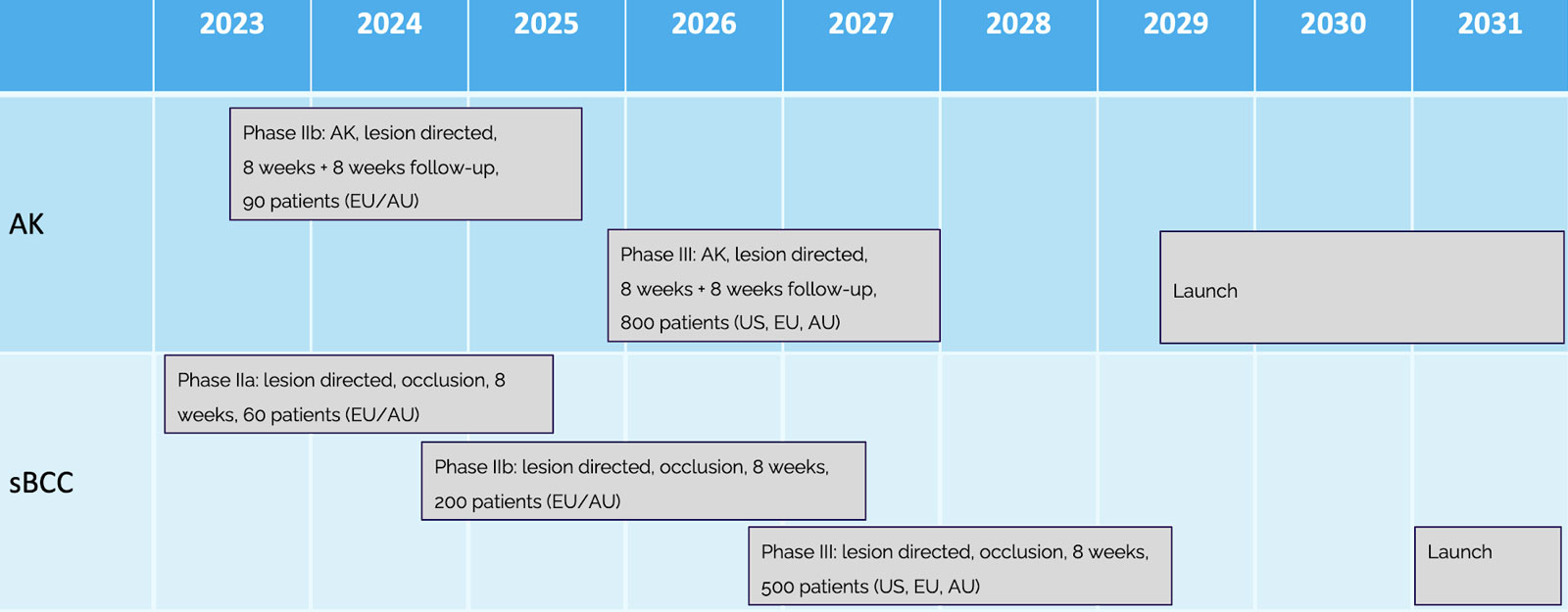
Next clinical trials designed to demonstrate the full potential of AVX001 in AK and BCC
The planned clinical studies include an AK phase IIb study with a lesion directed therapy treating up to 8 weeks and a phase IIa in basal cell carcinoma (BCC) treating up to 8 weeks under occlusion.
In order to extend the treatment period up to 8 weeks in both AK and BCC, a preclinical extended safety period of tolerability is planned early prior to the next clinical studies. In parallel we will produce the study medicine and prepare the clinical trial applications.
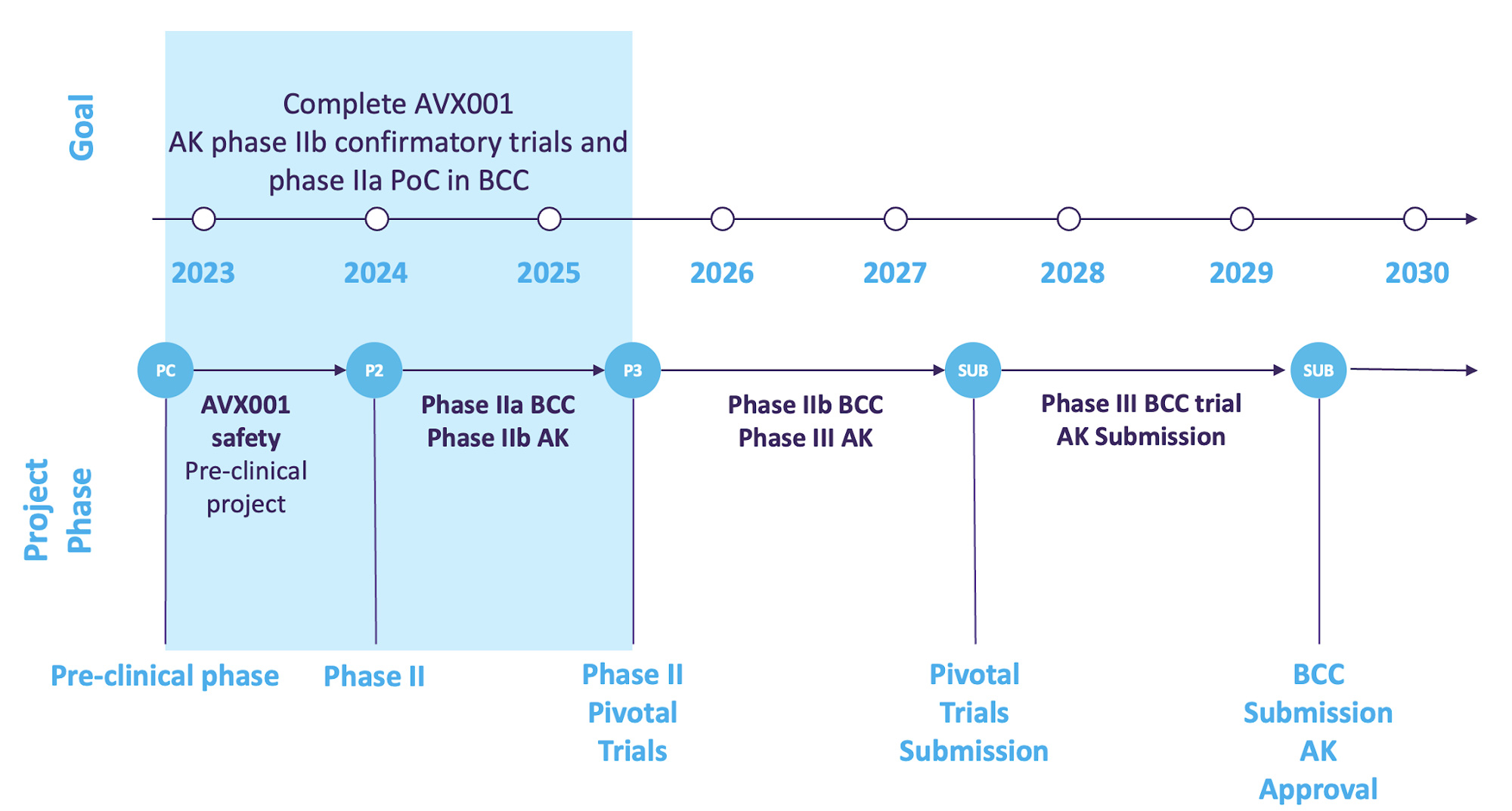
Roadmap with clear goal to build value of AVX001 towards exit
Roadmap with clear goal to build value of AVX001 towards exit.
A clear road map is based on co-development and exit:
2023: Partner discussions ongoing aiming at outlicensing and co-development.
2023: Additional skin tolerability studies to extend the treatment period, produce study medicine and prepare the clinical trial applications.
2024: First patient in phase IIb in AK and IIa in BCC clinical trials.
2025: Key results from the clinical trials.
An opportunity for novelty in the dermato-oncology market
The non-melanoma skin cancer market is substantial and dominated by specialty pharma companies with a dedicated focus on skin diseases. The market is generally characterized by well-established drugs, lack of innovation and a large number of patients with a clear need for better treatment options. Existing drugs are associated with significant drawbacks and there is a large unmet need for new drug-treatments that are safe, efficacious and convenient. Chemotherapies (e.g. 5FU) causes damage of the genes inside the nucleus of cells that are dividing and can cause severe skin reactions. Other treatments stimulate the immune system to destroy cancer cells (e.g. topial imiquimod). Several medical procedures are common in the treatment of AK (e.g. liquid nitrogen) and BCC (surgery) – these therapies are generally effective but often result in pain and severe scarring which compromise the patients’ quality oflife. These procedures often have to be repeated several times due to recurrence of the lesions.
AVX001 inhibits cPLA2α, a key enzyme which causes release of arachidonic acid resulting in activation of multiple inflammatory and proliferative processes play a fundamental role in cancer cells and their micro environment, hence acting on several key hallmarks of cancer growth. The cPLA2α inhibitor AVX001 has been shown to exhibit effect in preclinical and clinical studies with a better safety profile over existing treatments and medical procedures.
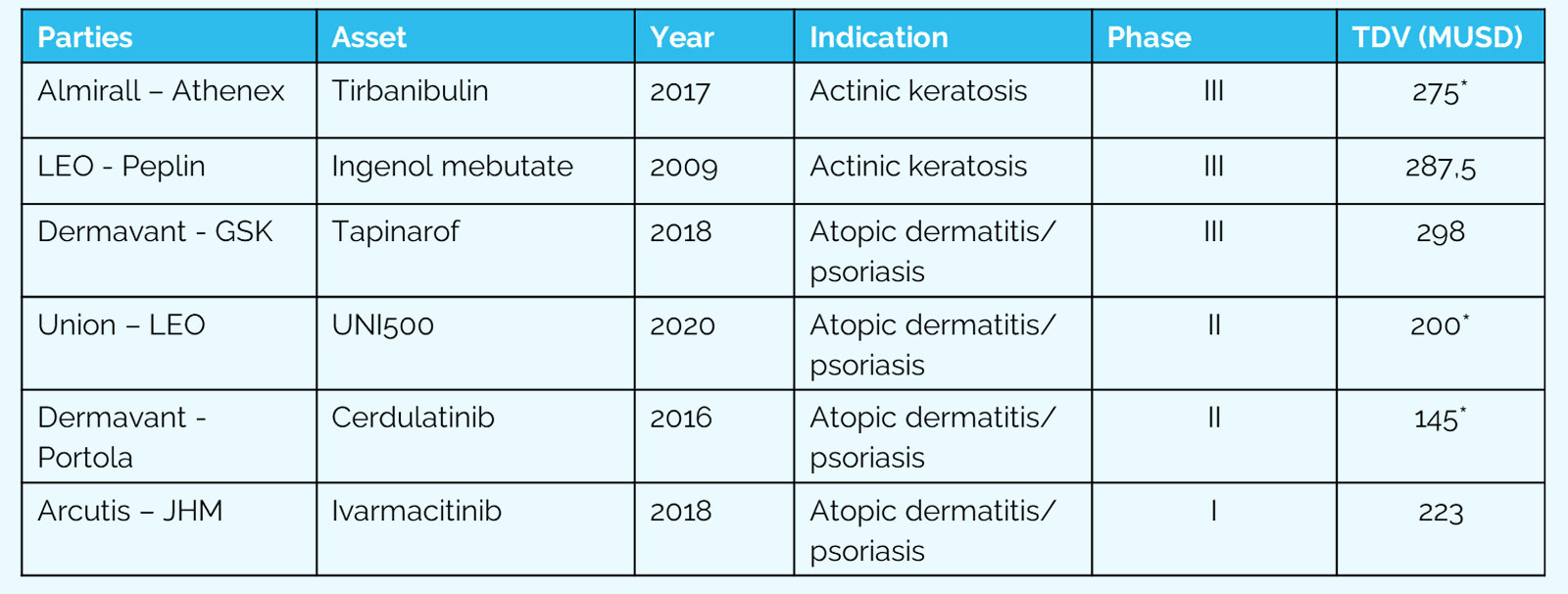
Exit by outlicensing and partnering deals
We are very familiar with the commercial players in dermatology and we are in active dialogue with potential partners which can either acquire AVX001 or can be a valuable co-development partner sharing the risks of the planned clinical trials. We are seeking global and regional license agreements. We aim to outlicense or partner AVX001 to one or several commercial companies before the next clinical Phase IIb in AK and Phase IIa in sBCC. The two clinical trials are designed to demonstrate the potential of the new treatment in both indications and to significantly increase the commercial value of the asset. This is a well-established commercial strategy for small and agile companies in the pharmaceutical space and will generate income from upfront and milestone payments and from royalties from product sales.